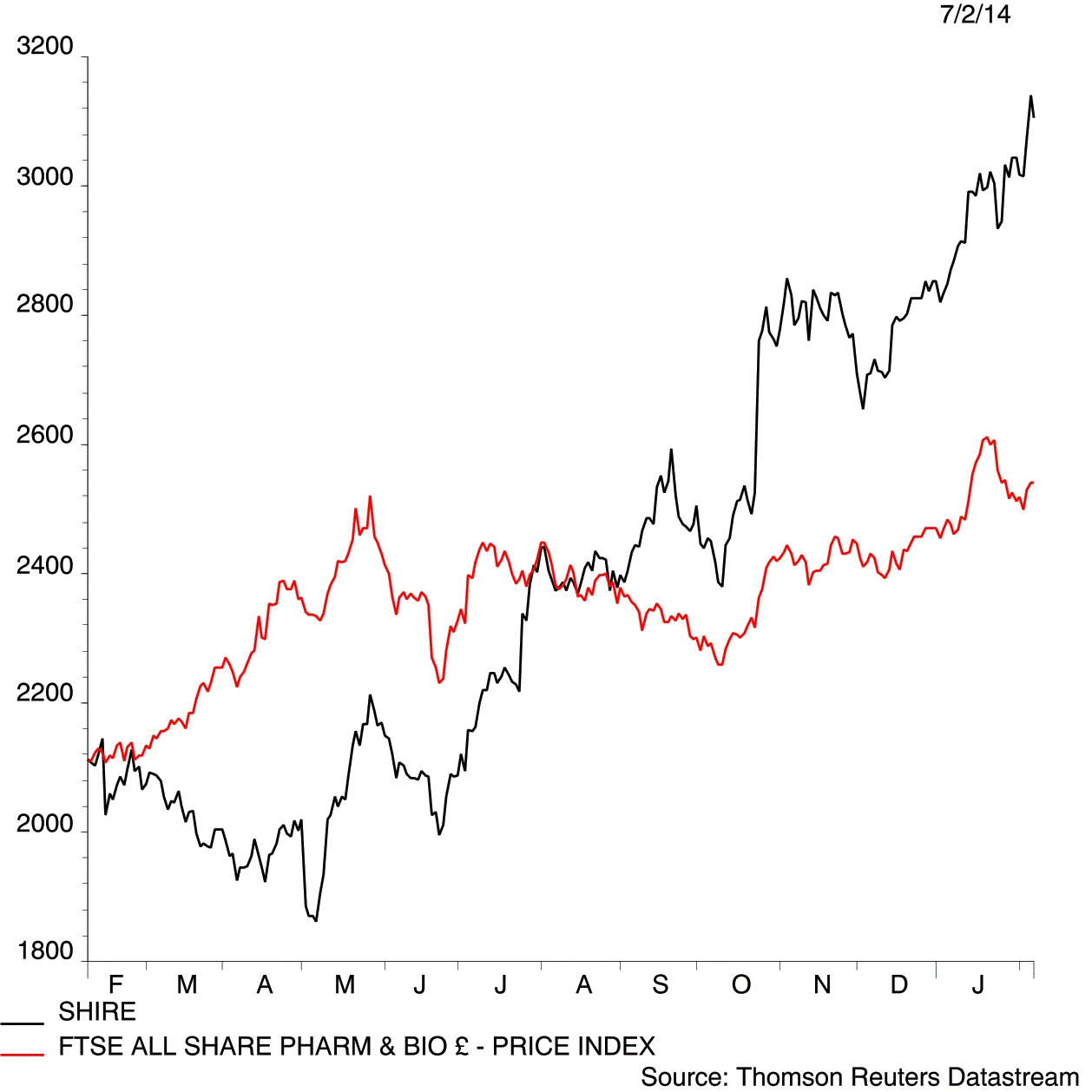
The UK’s third largest drug-maker Shire (SHP) highlights the development risk pharmaceuticals run as its depression drug fails in the late stages of clinical trials. The speciality medicine company slips 1.9% to £30.83 after failing to prove that its hyperactivity drug Vyvanse can also treat people with depression.
Vyvanse already helps those with attention deficit hyperactivity disorder (ADHD), but didn't improve on the placebo given to those with depression. Shire has abandoned any further attempt to develop the drug for that market, but is expected to seek to launch it in the US to treat those suffering from binge eating disorders.
Shire is big enough to contain the losses made on developing a failed drug, whereas some smaller pharma or biotech concerns would have lost their shareholders' money.
Indeed, Shire has other products in development such as its dry eye treatment Lifitegrast which showed some signs of success in December’s trial results announcement. It also has the cash to add treatments and technologies to bolster its offering, such as its $4.2 billion takeover of US biopharma company ViroPharma last month.
Shire announces its full-year results next week (13 Feb), where consensus expects a modest pre-tax profit growth to £992.3 million, up from £912 million a year ago. Its results have previously been hit by huge write downs and charges from selling some of its struggling assets.
Analyst Savvas Neophytou at Panmure Gordon remains positive. He sees Vyvanse as nothing more than a missed upgrading opportunity as he didn’t expect the trials to be successful. Neophytou is more interested in the ViroPharma acquisition, which has led him to upgrade his earnings per share forecasts for Shire in the next two years (2014/2015) to $2.64 and $2.88, respectively.