Management at Circassia (CIR) plans to transition from allergy-focused biotech into a speciality pharmaceutical after its main cat allergy treatment failed during clinical trials in June.
A focus on developing respiratory generics and selling other company’s treatments through its sales channels is the direction that Neil Woodford-backed business is now taking.
After a placebo outperformed its lead cat allergy treatment in phase III trials, grass allergy tests were quickly suspended.
Second phase dust mite tests are ongoing, however, as they are near completion. Results are scheduled for Spring next year.
Management has slashed the research and development (R&D) bill as it has no plans to invest in the allergy business. Instead, its £138 million cash will be used to expand the sales infrastructure and grow its asthma business.
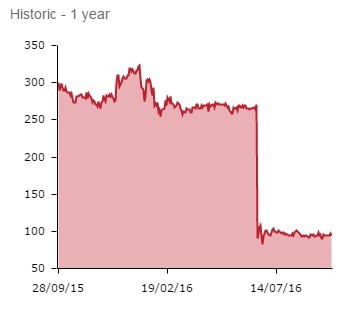
So Circassia, which saw 66% wiped off its value following June’s cat allergy failure, is no longer a high risk/high reward proposition. It is now a lower risk/revenue-led venture.
At the heart of this strategy is using its infrastructure to sell generic treatments and medicines as well as those belonging to third parties.
Circassia has 200 sales people, 140 of which are in the US, which should help force margins higher. Direct sales improved 35% during the period the six months to 30 June 2016.
Among its products approved for sale are Niox, an asthma device that assesses inflammation in the airways, and versions of GlaxoSmithKline’s (GSK) asthma inhaler Flixotide and asthma drug Advair.
Circassia has a copy of smokers’ cough drug Spiriva under development in its generic pipeline.
The jury appears to be out on the new strategy. Shares remained flat following the announcement, edging 0.6% lower to 94.3p.
This is a very different business to the one backed by shareholders 12 months ago.