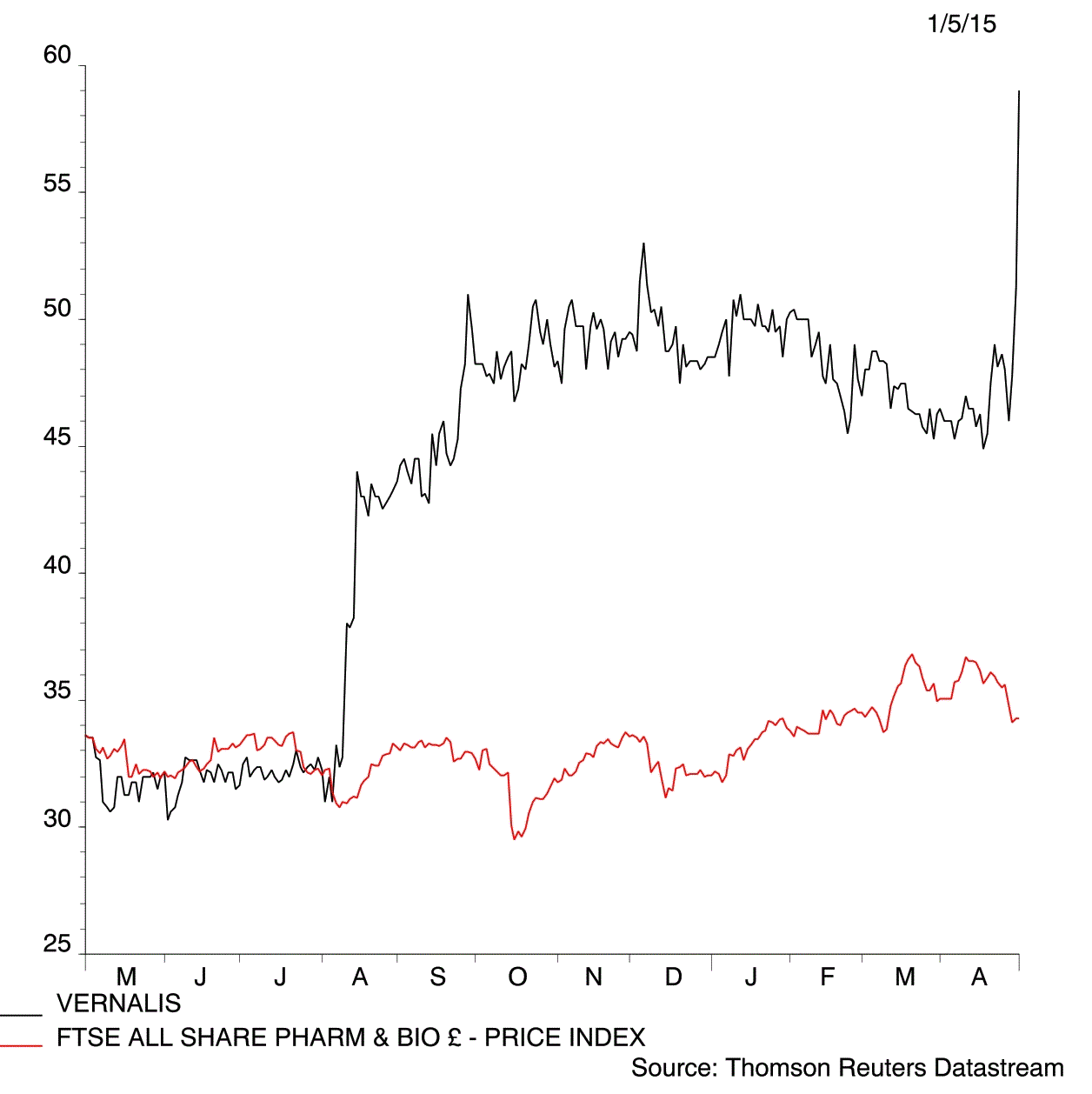
Drug developer Vernalis (VER:AIM) jumps 16.1% to 59.5p on the US regulator clearing its cough-cold treatment Tuzistra XR for salein a market worth $3 billion a year.
The extended-release treatment relieves the symptoms of the common cold, coughs and respiratory allergies. It will enter a market where up to 35 million prescriptions are written to treat these conditions each year.
Tuzistra, which has been developed by US outfit Tris Pharma, will be available from August following the Food & Drug Administration’s (FDA) decision.
The codine and antihistamine formulation means Tuzistra will be targeting 37% of the overall market, a $1.7 billion opportunity, according to analysts at Canaccord Genuity.
‘The Tuzistra XR approval is the first step in the transformation of Vernalis into a speciality pharma and we expect a very positive reaction from the shares following the reduction in risk,’ Canaccord Genuity said in a note. ‘Well capitalised with a further four products in the pipeline, we consider Vernalis a highly attractive investment opportunity.’
The price target has been upped to 92p from 66p.
The FDA’s decision has transformed Vernalis from a developer into a commercial drug company and with four other extended-release candidates in its pipeline more positive news could be on the way.